February Kids’ Corner
from the Gale River Cooperative Preschool
Why do we use salt on roads in winter?
Have you gotten stuck behind a snow plow this winter? If you have, you’ve probably noticed that many of the plows aren’t just pushing snow off the road, they’re also dropping stuff onto the road as they go! If you look closely, you might see that the stuff looks like small crystals, and they are! Salt crystals!
Lots of people use salt on roads during the winter to keep them less icy and safe for driving. This salt isn’t the same as the salt in the kitchen though, so don’t ever eat it!
Salt helps keep us safe on the road by stopping ice from forming in freezing weather. It does this by getting in between water molecules (you can think of water molecules as tiny bits of water) and stopping them from grabbing onto each other to form solid ice.
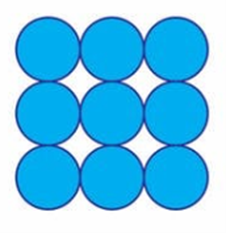
When water gets cold enough it clings to itself tightly; so tightly that it becomes a solid instead of a liquid. You can think of an ice cube as a bunch of water molecules bunching together so tightly that they don’t come apart, like in the picture to the right.
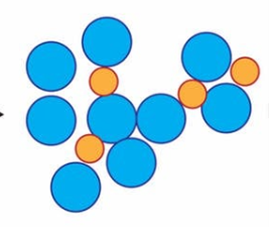
When salt is added to water, however, it gets in the way of the water bunching together and forming ice, like in the picture to the left. When salt is in between water molecules it has to be much colder for ice to form. This means that water on the road that would have turned to ice will stay as liquid water when the roads are covered in salt.
Image (Julie Pollock, CC BY-SA 4.0 <https://creativecommons.org/licenses/by-sa/4.0>, via Wikimedia Commons)
Salt can make our roads safer, but there are also some downsides to using it.
For one, have you noticed that your car gets covered in white grime in the winter? Part of that grime is the salt getting kicked up from the road and sticking to your car. Closing the car door or reaching into the car and getting that white powdery gunk all over your jacket and pants in the winter is the worst!
Another problem with salt is that it doesn’t stay on the road. Salt gets washed off the road and into water, forests, yards, etc; causing problems for plants and animals.
Salt can also be tough for pets. Have you seen dogs walking around in booties like these in the winter? They might just have very cold toes, but often dog owners will buy these shoes to protect their paws from the salt on roads and sidewalks.
Your Turn
You can experiment with how salt affects ice formation. All you need is water, salt, and a freezer (or you could put your experiment outside since it’s winter!)
Find an ice cube tray or container(s).
Set up at least three different sections: one with no salt, one with some salt, and one with lots of salt.
Make sure to keep track of which tray/container has which salt
Put them outside/in the freezer and check on them every couple of hours.
What do you notice about how long it takes the water to freeze? Does all the water freeze?
STTTRREEETTTCHHH Your Knowledge (Bonus)
Have you noticed that trucks are often putting salt on roads before the snow/ice even starts? Given what you know about salt and ice, why do you think it’s important that towns pre-treat the roads with salt rather than waiting until after they’re already icy?
If you already had ice in your driveway would you cover it with sand or salt? Why?